Abstract
Background:
Current prognostic models in primary myelofibrosis (PMF) target overall survival (OS) and utilize MIPSS70 (mutation-enhanced international prognostic scoring system for transplant-age patients), MIPSS70+ version 2.0 (karyotype-enhanced MIPSS70) and GIPSS (genetically-inspired prognostic scoring system, which is based on mutations and karyotype) (JCO 2018;36:310; JCO doi: 10.1200/JCO.2018.78.9867; Leukemia. 2018;doi:10.1038/s41375-018-0107). In the current study, we used logistic regression statistics to identify risk factors for leukemic transformation (LT) within 5 years of diagnosis/referral (i.e. early events) and also performed Cox regression analysis of overall leukemia-free survival (LFS).
Methods:
Study patients were recruited from the Mayo Clinic, Rochester, MN, USA. Diagnoses of LT and chronic phase PMF were confirmed by both clinical and bone marrow examinations, in line with the 2016 World Health Organization criteria (Blood. 2016;127:2391); specifically, LT required presence of ≥20% blasts in the peripheral blood (PB) or bone marrow (BM) (Blood 2016;127:2391). Statistical analyses considered clinical and laboratory data collected at the time of initial PMF diagnosis or Mayo Clinic referral point. Logistic regression statistics was used to identify predictors of LT at 5 years from initial diagnosis/referral; in the particular method, patients with documented LT within 5 years were "uncensored" while those followed up for at least 5 years, without developing LT, were "censored"; the analysis excluded patients without LT and not followed for at least 5 years. In addition, Cox regression analysis was performed to identify risk factors for overall LFS. The JMP® Pro 13.0.0 software from SAS Institute, Cary, NC, USA, was used for all calculations.
Results:
1,306 patients with PMF (median age 65 years; 63% males) were included in the current study; MIPSS70+ version 2.0 risk distribution was 20% very high risk, 41% high risk, 19% intermediate risk, 16% low risk and 4% very low risk. 149 (11%) patients were documented to experience LT, and compared to the remaining patients (n=1157), they were more likely to be males (p=0.02) and mutated for ASXL1 (p=0.01), SRSF2 (0.001) and IDH1 (0.02) and present with higher risk MIPSS70+ version 2.0 (p=0.02).
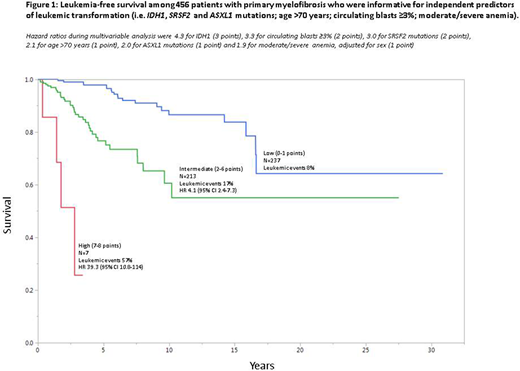
Multivariable logistic regression identified the following as predictors of LT in the first 5 years of disease: IDH1 mutation (odds ratio; OR 78.4), very high risk (VHR) karyotype (OR 57.6), ASXL1 mutation (OR 15.1), age >70 years (OR 13.3), SRSF2 mutation (OR 8.5), male sex (OR 6.9), PB blasts ≥3% (OR 5.4), presence of moderate or severe anemia, adjusted for sex (OR 3.6) and constitutional symptoms (OR 3.1). On Cox regression analysis, the following were associated with inferior LFS: IDH1 mutation (HR 4.3), PB blasts ≥3% (HR 3.3), SRSF2 mutation (HR 3.0), age >70 years (HR 2.1), ASXL1 mutation (HR 2.0) and presence of moderate or severe anemia, adjusted for sex (HR 1.9). Subsequently, HR-based risk point allocation resulted in highly discriminating LT predictive model with HR (95% CI) of 39.4 (10.8-114) for high risk and 4.1 (2.4-7.3) for intermediate risk (Figure 1).
Conclusions:
The current study identifies IDH1 mutation as a main predictor of LT in PMF. Our study also implicates SRSF2 and ASXL1 mutations and VHR karyotype as other genetic markers of early LT. Other independent contributors of early LT and inferior LFS, overall, included PB blasts ≥3%, moderate to severe anemia and older age. We provide LT prediction model, based on these variables, with leukemia risk ranging from 8% to 57%.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.